Due to the resource-abundance, the low standard redox potential of potassium and high conductivity of K+-based electrolyte, potassium-ion batteries (PIBs) have emerged as a compelling complement for large-scale energy storage applications. Particularly, considering the cost-effectiveness and environmental-friendliness, carbon materials stand out as the most promising candidates for PIBs anode. However, most research into carbon materials has focused on structural design and performance optimization for pursuing one or several parameters, rather than considering the holistic performances and practical requirements. Therefore, it is of great significance for the development of PIBs to construct carbon anode materials with high performance and commercial application. In view of different potassium storage behaviors, Professor Fei Xu group (State Key Laboratory of Solidification Technology, School of Materials Science and Engineering, Northwestern Polytechnical University) with the cooperation of Prof. Kaskel (Technische Universität Dresden), puts forward the opinion that further research must refocus to balance intercalation vs. surface-driven charge storage mechanisms in designing carbon structures, aiming at the construction of carbon materials with the advantages of high initial Coulombic efficiency (ICE), large capacity, decent rate performance and enhanced cyclability with a plateau-dominated K+ storage behavior. Meanwhile, the compatibility between the carbon electrode and electrolyte, and the cells assembly technologies under realistic conditions should be considered.
Several features are shown in this work:
1. Depending on the microstructure and discharge behavior of carbons, several charge storage mechanisms have been proposed including intercalation, adsorption, pore filling etc. Generally, two main mechanisms have been identified for the charge storage contribution: a diffusion-controlled intercalation process and a surface-driven capacitive process. The intercalation process is based on K+ intercalation/deintercalation into/from interlayers of graphitic carbon layers, and thus the storage capacity relies on ionic state, interlayer structure and so on. The intercalation process involves diffusion of K+ into graphite layers, which is sluggish with high volume expansion and collapse of the graphitic structure, thus causing insufficient rate capability and poor cyclic stability. The surface-driven capacitive K+ storage mainly happens at the surface or near surface region and does not pose damage to the electrode material. Thus, the storage capacity is related to specific surface area, intrinsic carbon defect/edges and heteroatom-doped functional sites within carbon materials.
2. Rational balancing of surface-driven and intercalation mechanism is desirable to incorporate the merits of high ICE, low-potential plateau, remarkable cyclability and rate capability. Currently, the key issue is to rationally control the crystalline graphitic structure and disordered microcrystallite nanodomains in the carbons. Various approaches have been adopted including (1) adjusting carbonization temperature, (2) adopting two or more precursors with distinct properties, and (3) designing pore/nanostructure in graphitic materials.
3. For carbon anode materials, electrolyte adaption is also important for enhancing the electrode stability and thus K storage performances, especially for those intercalation-controlled graphitic carbons showing large volume variation. Thus, to avoid the cracking of solid-electrolyte-interphase (SEI) layer under stress from volume change and impede further passivation and excessive side reactions, the selection of electrolyte should permit the as-formed SEI layer to be mechanically robust and/or help to reduce the volume variation. According to these, this paper briefly analyzes the electrolyte selection about carbon structures with different K storage mechanisms, including the solute, solvent and concentration types of potassium ion salts and solvents and the concentration of electrolyte.
The work was published in the international journal Advanced Energy Materials (DOI: https://doi.org/10.1002/aenm.202100856), under the title: “Perspective on carbon anode materials for K+ storage: balancing the intercalation-controlled and surface-driven behavior”. The corresponding authors are Professor Fei Xu, the State Key Laboratory of Solidification Technology, and Professor Stefan Kaskel, Inorganic Chemistry I Technische Universität Dresden.
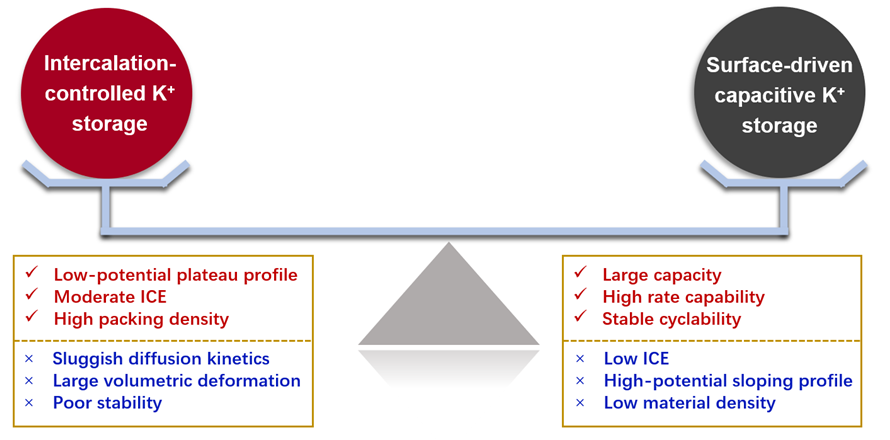
Figure 1. Schematic illustration of balancing the intercalation-controlled and surface-driven capacitive K+ storage with their respective strengths and weaknesses.
Author: Fei Xu
Reviewer: Wei Liu ,Hongqiang Wang